This opinion piece by Bill Marler first appeared on Marler Blog and is reposted here with the author’s permission.
As I wrote last Spring, I have tried to steer clear of politics with respect to the Felon in Chief, but this is nuts, or at least peanuts. According to yet another edict from the White House, it is now the policy of the United States that:
(a) Criminal enforcement of criminal regulatory offenses is disfavored.
(b) Prosecution of criminal regulatory offenses is most appropriate for persons who know or can be presumed to know what is prohibited or required by the regulation and willingly choose not to comply, thereby causing or risking substantial public harm. Prosecutions of criminal regulatory offenses should focus on matters where a putative defendant is alleged to have known his conduct was unlawful.
(c) Strict liability offenses are “generally disfavored.” United States v. United States Gypsum, Co., 438 U.S. 422, 438 (1978). Where enforcement is appropriate, agencies should consider civil rather than criminal enforcement of strict liability regulatory offenses or, if appropriate and consistent with due process and the right to jury trial, see Jarkesy v. Securities and Exchange Commission, 603 U.S. 109 (2024), administrative enforcement.
(d) Agencies promulgating regulations potentially subject to criminal enforcement should explicitly describe the conduct subject to criminal enforcement, the authorizing statutes, and the mens rea standard applicable to those offenses.
Typically, every crime has two elements—a bad act and a culpable state of mind (mens rea, which generally means intent or recklessness). Section 333(a)(1) of the FDCA, the misdemeanor provision, is noteworthy because it creates one of the few true strict liability crimes in federal criminal law. That is, the government does not need to prove a state of mind to obtain a conviction. If a food product is misbranded or adulterated and is distributed into the channels of interstate commerce, a crime has been committed. Depending upon the nature of the conduct, a violation of the Food, Drug, and Cosmetic Act may be a felony or a misdemeanor. Misdemeanor violations of section 331 are punishable by a maximum prison sentence of one year and a maximum fine of $100,000.
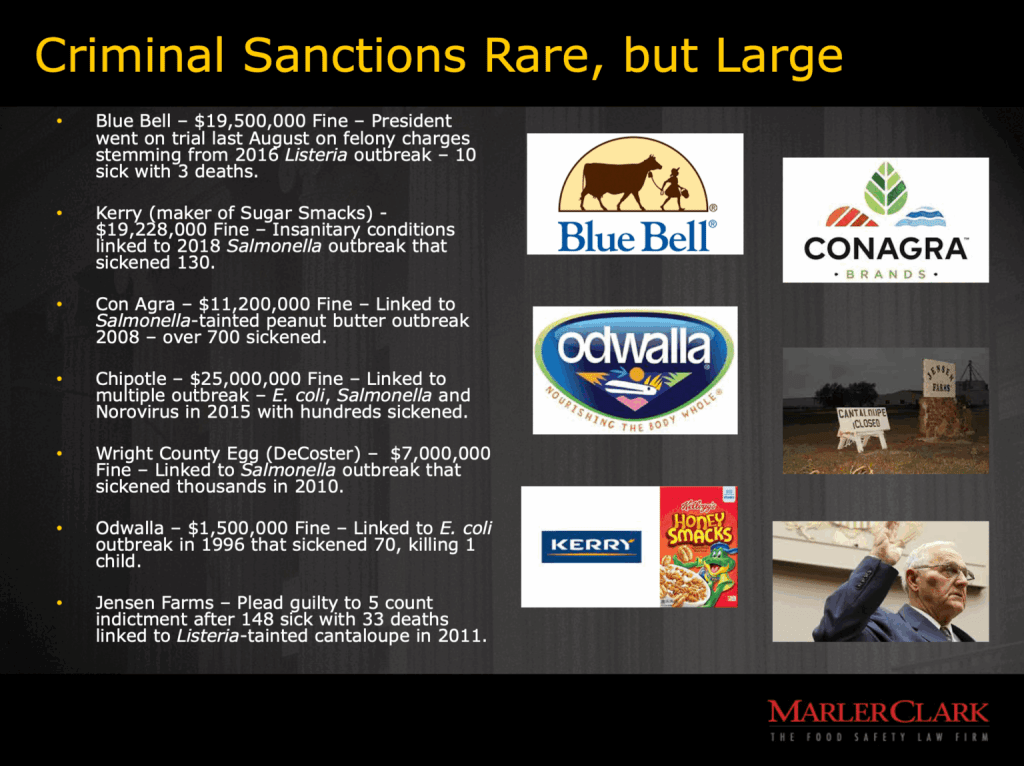
Perhaps the folks at the Peanut Corporation of America would still be prosecuted as a felony, but they certainly argued that they did not know the peanut butter was tainted with Salmonella. However, if this new policy stands it is not likely the Blue Bell, Kerry, Con Agra, Chipotle, Wright County, Odwalla nor Jensen Farms would have been prosecuted, and it is likely that few prosecution will occur in the future.
Even if they had an appetite to prosecute, there will be no one there to do the work. According to Sarah N. Lynch at Reuters:
A Justice Department unit that handles criminal and civil enforcement of U.S. food and drug safety laws is being disbanded as part of an ongoing cost-cutting campaign by President Donald Trump’s administration…
About 215 people work for the Consumer Protection Branch, part of the Justice Department’s Civil Division, including attorneys, support staff and law enforcement agents…
Although it is located in the Civil Division, the Consumer Protection Branch is an unusual office because its work involves a hybrid of criminal prosecutions and civil enforcement.
It handles criminal cases to enforce the Food, Drug and Cosmetic Act, a federal law that makes it a crime to sell or distribute adulterated or misbranded food or drugs. It also enforces statutes for the Federal Trade Commission and the Consumer Product Safety Commission…
The Consumer Protection Branch has been at the heart of some high-profile cases…
Prosecutors from the branch also brought the criminal case against former executives at Peanut Corporation for crimes that led to a 2009 outbreak involving more than 700 cases of salmonella poisoning.
We live in interesting times.



